Introduction
Newborn calves have an underdeveloped immune system and no circulating maternal antibodies, leaving them highly susceptible to infectious diseases. Unlike humans, where passive immunity is transferred through the placenta, the synepitheliochorial placenta of cattle prevents the transfer of immunoglobulins from the dam to the fetus (Peter, 2013). As a result, calves are born without humoral immunity and rely entirely on colostrum intake for passive immunity.
Immunoglobulins and their role in calf immunity
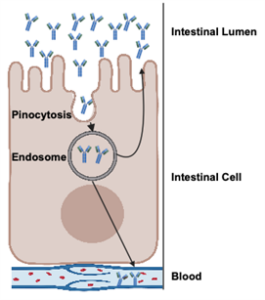
Figure 1. Process of immunoglobulin absorption through pinocytosis in the intestinal cell.
What immunoglobulin?
Although colostrum contains other immunoglobulins, such as IgM and IgA, IgG is the predominant antibody (Figure 2) and the primary focus of research due to its central role in passive immunity. Once absorbed, IgG neutralizes pathogens, enhances opsonization, and supports adaptive immune development (Janeway et al., 2001). Additionally, IgG can be re-secreted into the intestine, contributing to mucosal immunity alongside IgA (Besser et al., 1988; Ulfman et al., 2018) (as shown in Figure 1)
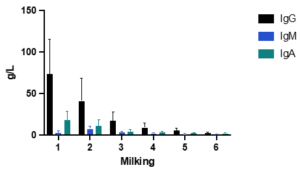
Figure 2. Postparturient colostral IgG, IgA, and IgM concentrations for 6 milkings after calving at 12 hours intervals. Data from Stott et al. (1981).
Effects of passive immunity
Short-term effects
Failure of transfer of passive immunity (FTPI) is typically defined as serum IgG < 10 g/L in a calf at 24 to 36 hours of age (Weaver et al., 2000). Using this threshold, Raboisson et al. (2016) conducted a meta-analysis of 10 studies and found that dairy calves with FTPI had:
- 2.12 times higher risk of mortality
- 1.75 times higher risk of respiratory disease
- 1.51 times higher risk of diarrhea
- 1.91 times higher risk of overall morbidity
- 81 g/day lower average daily gain
Cumulatively, based on the study results, the estimated economic impact of FTPI was found to be $89.27 CAD per case. Similarly, Abdallah et al. (2022) conducted a meta-analysis on non-replacement dairy calves (veal or dairy-beef) using the same FTPI threshold (< 10 g IgG/L) and found that affected calves had:
- 2.46 times higher odds of mortality
- 3.03 times higher odds of diarrhea
More recent research suggests that higher thresholds should be used to define adequate passive immunity. Lombard et al. (2020), through expert consensus, concluded that the traditional 10 g/L cutoff is too low and that achieving higher serum IgG levels is critical for optimal calf health. The recommended thresholds for serum IgG concentrations, total protein, and Brix % are outlined in Table 1.
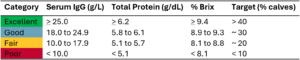
Table 1. Consensus serum IgG concentrations, total protein, and Brix %, along with the suggested targets by Lombard et al. (2020).
Multiple studies have confirmed the benefits of achieving higher passive immunity thresholds. Sutter et al. (2023) analyzed serum total protein data from 3,434 dairy calves sampled between 2 to 7 days of age on a commercial dairy farm. They found that calves with excellent passive immunity (vs. poor) had:
- 50% lower hazard for respiratory disease
- 50% lower hazard for overall morbidity
- 60% lower hazard for mortality
- 0.04 kg/day higher average daily gain
Crannell and Abuelo (2023), also had similar findings. Analyzing serum total protein records from 4,336 dairy calves sampled between 2 to 7 days of age on a commercial dairy farm, they reported that calves with excellent passive immunity (vs. poor) had:
- 33% lower hazard for diarrhea
- 28% lower hazard for respiratory disease
- 34% lower hazard for overall morbidity
- 77% lower hazard for mortality
Long-term effects
Few studies have examined the long-term impacts of passive immunity. DeNise et al. (1989) analyzed serum IgG levels in 1,000 calves sampled between 24 to 48 hours of age and found that for every 1 g/L increase in IgG, first-lactation milk yield increased by 8.5 kg. Additionally, calves with IgG < 12 g/L had the highest rates of culling for low production in their first lactation and increased mortality from birth to 180 days.
More recently, Crannell and Abuelo (2023) applied the Lombard et al. (2020) passive immunity thresholds and found that calves in the excellent category (vs. poor) had:
- 2.78 times higher hazard of being inseminated
- 2.22 times higher hazard of becoming pregnant as a heifer
- 1.32 times higher hazard of calving for the first time
Similarly, Faber et al. (2005), although not directly measuring IgG, reported that calves fed 4 L of colostrum at birth produced 955 kg more milk in their first lactation and 1,652 kg more in their second lactation compared to those receiving 2 L of colostrum.
Going beyond passive immunity
Although IgG and passive immunity have been the primary focus, colostrum contains a variety of bioactive compounds that influence immune system development and gut health (Blum and Hammon, 2000; Fischer-Tlustos et al., 2021). Feeding colostrum soon after birth supports early microbial colonization, promoting beneficial bacteria while reducing potential pathogens (Malmuthuge et al., 2015). Additionally, Fischer-Tlustos et al. (2020) reported that earlier colostrum intake improved villi height and crypt depth, increasing the surface area for nutrient absorption. While IgG is often emphasized, its benefits may be closely linked to other bioactive components that contribute to overall calf health.
Take away messages
Colostrum is essential for calf immunity, as newborns are born without maternal antibodies and rely entirely on passive transfer for protection. Because IgG absorption declines rapidly, with significantly reduced permeability after 12 hours, timely colostrum feeding is critical. Higher passive immunity improves short-term health by reducing the risk of mortality, respiratory disease, and diarrhea while also enhancing growth. Long-term benefits include improved first-lactation milk yield, lower culling rates, and better reproductive performance. Recent research suggests that the traditional 10 g/L IgG threshold is too low, and achieving higher passive immunity levels is necessary for optimal health and productivity. Ensuring calves receive a sufficient quantity of high-quality colostrum immediately after birth is essential for their health, growth, and long-term success.
Dave Renaud, DVM PhD, Associate Professor, University of Guelph
References
Abdallah A, Francoz D, Berman J, Dufour S, Buczinski S. Association between transfer of passive immunity and health disorders in multisource commingled dairy calves raised for veal or other purposes: Systematic review and meta-analysis. Journal of Dairy Science. 2022 Oct 1;105(10):8371-86.
Besser TE, Gay CC, McGUIRE TC, Evermann JF. Passive immunity to bovine rotavirus infection associated with transfer of serum antibody into the intestinal lumen. Journal of Virology. 1988 Jul;62(7):2238-42.
Blum JW, Hammon H. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livestock Production Science. 2000 Oct 1;66(2):151-9.
Crannell P, Abuelo A. Comparison of calf morb
DeNise SK, Robison JD, Stott GH, Armstrong DV. Effects of passive immunity on subsequent production in dairy heifers. Journal of dairy science. 1989 Feb 1;72(2):552-4.
Faber SN, Faber NE, McCauley TC, Ax RL. Case study: effects of colostrum ingestion on lactational performance 1. The professional animal scientist. 2005 Oct 1;21(5):420-5.
Fischer-Tlustos AJ, Lopez A, Hare KS, Wood KM, Steele MA. Effects of colostrum management on transfer of passive immunity and the potential role of colostral bioactive components on neonatal calf development and metabolism. Canadian Journal of Animal Science. 2021 Feb 24;101(3):405-26.
Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The distribution and functions of immunoglobulin isotypes. InImmunobiology: The Immune System in Health and Disease. 5th edition 2001. Garland Science.
Lombard J, Urie N, Garry F, Godden S, Quigley J, Earleywine T, McGuirk S, Moore D, Branan M, Chamorro M, Smith G. Consensus recommendations on calf-and herd-level passive immunity in dairy calves in the United States. Journal of dairy science. 2020 Aug 1;103(8):7611-24.
Malmuthuge N, Chen Y, Liang G, Goonewardene LA. Heat-treated colostrum feeding promotes beneficial bacteria colonization in the small intestine of neonatal calves. Journal of dairy science. 2015 Nov 1;98(11):8044-53.
Peter AT. Bovine placenta: a review on morphology, components, and defects from terminology and clinical perspectives. Theriogenology. 2013 Oct 15;80(7):693-705.
Raboisson D, Trillat P, Cahuzac C. Failure of passive immune transfer in calves: A meta-analysis on the consequences and assessment of the economic impact. PloS one. 2016 Mar 17;11(3):e0150452.
Stott GH, Marx DB, Menefee BE, Nightengale GT. Colostral immunoglobulin transfer in calves I. Period of absorption. Journal of dairy science. 1979 Oct 1;62(10):1632-8.
Stott GH, Fleenor WA, Kleese WC. Colostral immunoglobulin concentration in two fractions of first milking postpartum and five additional milkings. Journal of dairy science. 1981 Mar 1;64(3):459-65.
Sutter F, Venjakob PL, Heuwieser W, Borchardt S. Association between transfer of passive immunity, health, and performance of female dairy calves from birth to weaning. Journal of Dairy Science. 2023 Oct 1;106(10):7043-55.
Ulfman LH, Leusen JH, Savelkoul HF, Warner JO, Van Neerven RJ. Effects of bovine immunoglobulins on immune function, allergy, and infection. Frontiers in nutrition. 2018 Jun 22;5:52.
Weaver DM, Tyler JW, VanMetre DC, Hostetler DE, Barrington GM. Passive transfer of colostral immunoglobulins in calves. Journal of veterinary internal medicine. 2000 Nov;14(6):569-77.
