Introduction
Respiratory disease is one of the most common and economically significant health challenges affecting preweaned dairy calves. It accounts for a substantial proportion of antibiotic treatments, veterinary costs, and calf losses on farms, and it remains a persistent barrier to achieving optimal calf health and productivity.
How common is it?
In a Canadian study involving 74 dairy farms and over 7,800 calves, nearly 30% of calves were treated at least once during the preweaning period with an antibiotic, and respiratory disease was the most frequently cited reason for treatment. Specifically, it accounted for 54% of all recorded antibiotic treatments (Uyama et al., 2022). Similarly, data from a multi-state U.S. study of more than 2,500 calves reported that one-third of calves experienced at least one health event, with respiratory signs observed in 33% of sick calves. Among those with respiratory signs, 88% received antibiotics. The same study reported an overall preweaning mortality rate of 5% percent, with respiratory disease responsible for 14% of deaths and an additional 7% involving both respiratory and digestive causes (Urie et al., 2018).
What is the impact?
Respiratory disease during the preweaning period has serious consequences for both dairy heifer performance and in calves raised for beef or veal.
A recent meta-analysis evaluated the impact of preweaning respiratory disease on dairy heifers using data from 27 studies (Buczinski et al., 2021). Heifers diagnosed with respiratory disease during the preweaning period had 3 times higher odds of dying and 2 times higher odds of being removed from the herd, either through culling, sale, or death, before first calving compared to unaffected heifers. These heifers also gained 67 grams less per day during the preweaning phase and produced 121 kilograms less milk in their first lactation. Studies included in the meta-analysis also found that respiratory disease was associated with a delay in age at first calving by 8 to 14 days and a lower likelihood of surviving through the first lactation. Together, these findings illustrate the impact of early-life respiratory disease on dairy herd productivity.
In calves raised for veal or dairy-beef, the effects are also substantial. In a study of 3,519 veal calves followed through to slaughter, a single episode of respiratory disease was associated with an average reduction of 8 kilograms in hot carcass weight, lower fat cover, and a 6 times higher risk of mortality compared to calves without respiratory disease (Pardon et al., 2013). The impact worsened with repeated illness, with calves that experienced two or three or more episodes of respiratory disease had average reductions in hot carcass weight of 22 and 42 kilograms, respectively. Multiple episodes were also linked to poorer carcass quality and an increased likelihood of undesirable red meat color at slaughter.
Whether calves are destined for the milking herd or the beef supply chain, the consequences of respiratory disease are significant, underscoring the importance of targeted prevention and strong foundational immunity to protect calf health and performance.
What are the key factors to consider with prevention?
Housing and the environment
The environment in which calves are raised has a direct influence on their respiratory health. Well-managed housing can reduce pathogen exposure and support immune function, while poor conditions can increase the risk of respiratory disease through both direct and indirect stressors.
Bedding is a critical component of the calf’s environment. As bedding accumulates manure and forms a deep, wet pack, it contributes to a higher incidence of respiratory illness in preweaned calves (Donlon et al., 2023). This is likely due to both elevated bacterial load and the accumulation of ammonia, a pollutant produced from the microbial breakdown of urea in contaminated bedding and manure. Beyond bedding, other aspects of the housing environment also play a role. Proper ventilation helps control moisture and airborne contaminants, but excessive airspeed when the calf is outside of its thermoneutral zone can lead to chilling and increased respiratory disease (Donlon et al., 2023). Additionally, providing more than 35 square feet per calf has been shown to reduce airborne bacterial concentrations, likely by improving airflow and reducing animal density (Norlund and Halbach, 2019). The influence of these factors is summarized in Figure 1.
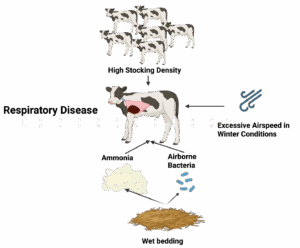
Figure 1. Influence of environmental and housing factors on the development of respiratory disease in calves.
Where does colostrum fit?
Colostrum, or more specifically achieving adequate passive immunity, is not always top of mind when discussing respiratory disease prevention. However, the evidence clearly shows that it plays a central role in reducing the risk of pneumonia in young calves.
A recent summary of eight studies on pneumonia in calves, most of which focused on dairy herds, found that calves with poor colostrum intake were much more likely to get respiratory disease. Specifically, the risk of respiratory disease was 1.6 times higher in calves that had failed transfer of passive immunity (Thompson and Smith, 2022). The authors estimated that 31 percent of respiratory disease cases in calves with failed transfer of passive immunity could be directly attributed to inadequate colostrum management. Looking more broadly, the median population attributable fraction was 17 percent, meaning that across an entire herd, nearly one in six pneumonia cases could be avoided by improving passive immunity in all calves, not just those at highest risk.
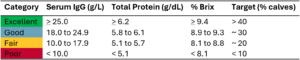
Recent research has also highlighted the value of achieving even higher levels of passive immunity than previously thought. Combining results from three studies representing 10,000 dairy heifers grouped by passive immunity levels (Crannell and Abuelo, 2023; Lombard et al., 2020; Sutter et al., 2023), treatment for respiratory disease occurred in 25 percent of calves with poor immunity, 18 percent with fair, 13 percent with good, and only 11 percent with excellent passive immunity. These findings show that aiming for excellent (> 25 g of IgG absorbed per L of serum), not just adequate, colostrum transfer can make a meaningful difference in preventing respiratory disease.
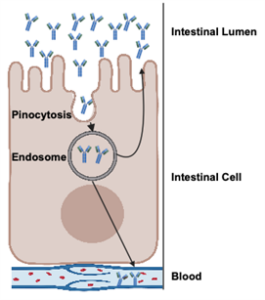
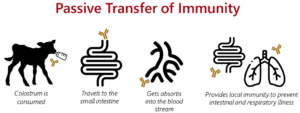
This protection is likely due to immunoglobulin G, the dominant antibody in colostrum. Once absorbed, IgG circulates in the blood, neutralizing pathogens and supporting the early immune response. In addition to its systemic role, IgG can also be transported back into mucosal surfaces, including the respiratory tract, where it helps block pathogens at the site of infection. Figure 2 highlights how IgG protects against respiratory disease. Colostrum also contains other immune-supporting compounds, like lactoferrin, cytokines, and growth factors, that further enhance early immune development and resilience against disease.
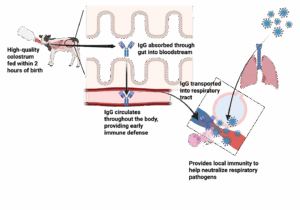
Figure 2. Pathway of how colostrum supports the prevention of respiratory disease.
Take Home Messages
Preventing respiratory disease in calves requires attention to both the environment and early-life care. Clean, dry bedding, good ventilation, and enough space help reduce exposure to airborne pathogens. Just as important, feeding high-quality colostrum soon after birth gives calves the protection they need to stay healthy. Together, these practices lower disease risk, reduce treatment needs, and support better long-term outcomes.
Dave Renaud DVM PhD, Associate Professor, University of Guelph
References
Buczinski S, Achard D, Timsit E. Effects of calfhood respiratory disease on health and performance of dairy cattle: A systematic review and meta-analysis. Journal of Dairy Science. 2021 Jul 1;104(7):8214-27.
Crannell P, Abuelo A. Comparison of calf morbidity, mortality, and future performance across categories of passive immunity: A retrospective cohort study in a dairy herd. Journal of Dairy Science. 2023 Apr 1;106(4):2729-38.
Donlon JD, McAloon CG, Hyde R, Aly S, Pardon B, Mee JF. A systematic review of the relationship between housing environmental factors and bovine respiratory disease in preweaned calves-Part 2: Temperature, relative humidity and bedding. The Veterinary Journal. 2023 Oct 1;300:106032.
Lombard J, Urie N, Garry F, Godden S, Quigley J, Earleywine T, McGuirk S, Moore D, Branan M, Chamorro M, Smith G. Consensus recommendations on calf-and herd-level passive immunity in dairy calves in the United States. Journal of dairy science. 2020 Aug 1;103(8):7611-24.
Nordlund KV, Halbach CE. Calf barn design to optimize health and ease of management. Veterinary Clinics: Food Animal Practice. 2019 Mar 1;35(1):29-45.
Pardon B, Hostens M, Duchateau L, Dewulf J, De Bleecker K, Deprez P. Impact of respiratory disease, diarrhea, otitis and arthritis on mortality and carcass traits in white veal calves. BMC Veterinary Research. 2013 Dec;9:1-4.
Raboisson D, Trillat P, Cahuzac C. Failure of passive immune transfer in calves: A meta-analysis on the consequences and assessment of the economic impact. PloS one. 2016 Mar 17;11(3):e0150452.
Sutter F, Venjakob PL, Heuwieser W, Borchardt S. Association between transfer of passive immunity, health, and performance of female dairy calves from birth to weaning. Journal of Dairy Science. 2023 Oct 1;106(10):7043-55.
Thompson AC, Smith DR. Failed transfer of passive immunity is a component cause of pre-weaning disease in beef and dairy calves: A systematic review and meta-analysis. The Bovine Practitioner. 2022 Dec 29;56(2):47-61.
Urie NJ, Lombard JE, Shivley CB, Kopral CA, Adams AE, Earleywine TJ, Olson JD, Garry FB. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. Journal of dairy science. 2018 Oct 1;101(10):9229-44.
Uyama T, Renaud DL, Morrison EI, McClure JT, LeBlanc SJ, Winder CB, de Jong E, McCubbin KD, Barkema HW, Dufour S, Sanchez J. Associations of calf management practices with antimicrobial use in Canadian dairy calves. Journal of Dairy Science. 2022 Nov 1;105(11):9084-97.